Cardiotoxicity Risk – A Step in the Right Direction

A recent paper from NTP showcases the application of mechanism-based NAMs for prediction of cardiotoxic potential.
In a previous post, Getting Back on Track with NAMs at FDA, we encouraged FDA to step up the effort to apply alternatives to animals for drug attrition. We have argued that emphasizing mechanism-based NAMs and integrating these into expert systems is the approach most likely to succeed.
The recent paper from Shagun Krishna, Brian Berridge and Nicole Kleinstreuer, “High Throughput Screening to Identify Chemical Cardiotoxic Potential”, takes a step in this direction. This study from the National Toxicology Program at NIEHS, takes advantage of data generated for the Tox21 and ToxCast programs which have sponsored compound testing on a broad range of human-based molecular and cellular assays in high throughput mode for more than a decade. For this analysis, potential mechanisms of cardiotoxicity (cardiac and cardiovascular) were grouped into six “failure modes”. These modes affect vasoactivity (dilation or constriction of blood vessels), contractility (inotropy) of the heart, heart rhythm or rate (changes in action potential), heart valves (valvulopathy), endothelial cells, coagulation, and cardiomyocytes (heart muscle or myocardium).

Image created in Canva by the author.
For each of the six failure modes, the research literature and pathway databases were queried to find associated targets and mechanisms. Using literature annotations, 314 of the Tox21/ToxCast assays (representing 40 targets) were associated with cardiovascular disease and cardiotoxicity. Data from >1300 chemicals (including some pharmaceuticals) that had been tested through these assays were analyzed a scoring method for cardiotoxicity potential (CardioToxPi) was developed.
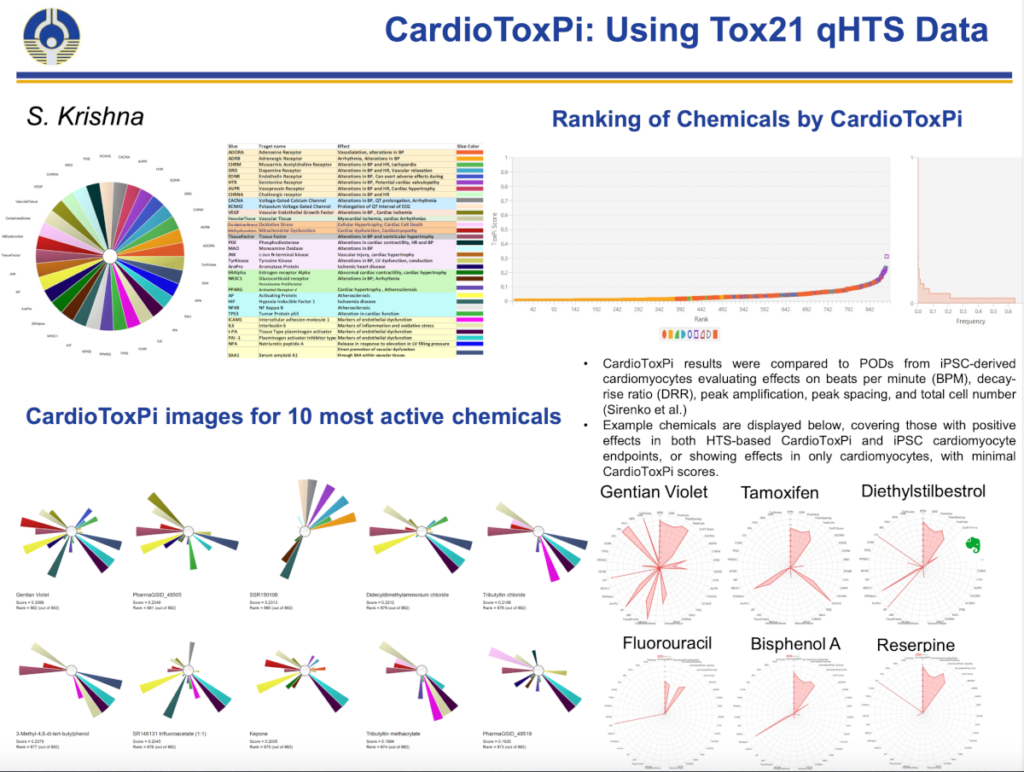 Image from the NICEATM update presentation at the ICCVAM Public Forum May 21, 2020, pdf here.
Image from the NICEATM update presentation at the ICCVAM Public Forum May 21, 2020, pdf here.
This effort represents a bottoms-up approach for prioritizing environmental chemicals for further testing based on best-available evidence from in vitro and human-based assay data. The idea being that chemicals interacting with multiple cardiotoxicity-related targets or pathways would be of greatest concern and therefore could be prioritized.
This study was somewhat limited in that it included only assay targets and mechanisms that had already been associated with one or more cardiovascular system failure modes. It was not designed to uncover new mechanisms of toxicity, but rather, to set a baseline for our current knowledge.
This work did effectively highlight some of the challenges for predicting cardiotoxicity. The large numbers of potential targets and mechanisms impacting different (and in some cases overlapping) failure modes is one challenge. Also, many chemicals of interest have activity on multiple targets (polypharmacology). This characteristic complicates interpretation of results. Finally, exposure levels have yet to be incorporated into the CardioToxPi score. This remains a challenge for environmental chemicals where exposure data is limited.
To apply this approach more broadly, we suggest future research to connect the identified targets, mechanisms and failure modes more directly to clinical measures and outcomes. Connecting assay results to testable clinical measures (e.g., blood pressure, EKGs, incidence of stroke, heart attack or sudden death) can help confirm mechanistic hypotheses that may emerge. Such efforts could also be useful for prioritizing mechanisms of concern (by their relative frequencies or clinical impact).
We can look to human cell-based phenotypic assays and more advanced tissue models to help build these connections, for example using Adverse Outcome Pathway frameworks. The six failure modes encompassing heart and blood vessel tissues, involve many cell types. These include cardiomyocytes, endothelial cells, smooth muscle cells, interstitial cell types such as fibroblasts as well as cells of the nervous and immune systems. To fully understand cardiotoxicity mechanisms will require incorporating tissue context. Tissue and cellular context is also important for general cell mechanisms such as mitochondrial dysfunction and oxidative stress.
Compared to environmental chemicals, pharmaceutical compounds have known exposures and are evaluated in controlled clinical trials. Thus, expanding this work to include more pharmaceutical compounds and their data will do much to advance our understanding of cardiotoxicity mechanisms.
Although there is a long road ahead, the first steps have been taken.
Photo by Ricardo Gomez Angel on Unsplash.






